Members of the Institute for Biomedical Engineering (IBE) in the Faculty of Engineering, Stellenbosch University, and other research groups in the Department of Mechanical and Mechatronic Engineering, have responded to the great demand for ventilators in South Africa created by the Covid-19 pandemic.
In addition to Government’s National Ventilator Project’s (NVP) aim to build 10 000 Rapidly Manufactured Continuous Positive Airway Pressure (RMCPAP) ventilators by the end of June, this research group hopes to design and build a Non-Invasive Ventilation System (NVS) that more closely represents traditional ventilators than a RMCPAP system. The NVS will be designed as to be built using only parts and materials that are readily available in large quantities or can be manufactured locally. South Africa has about 6 000 ventilators available in public and private hospitals.
“We are still in the prototype and early testing phase,” says Cornel de Jongh, a member of IBE and project leader of the NVS project. “We are currently finalising the matching of clinical requirements for the ventilator to engineering requirements by fine-tuning our feedback control protocols for the system. Until now we have utilised 3D printing to create mechanical components such as the Ambu bag effector (actuator), cradle as well as servo motor housing. Currently all the components are made to be modular to allow maximum adjustability and rapid change to the design should it be required. We have found, for example, that the shape and width of the Ambu bag effector plays a significant role in the effective delivery of Tidal Volume (volume of air per breath) to the patient. The prototype enclosure of the system is currently being manufactured by CHG Engineering in Somerset West. Furthermore, we have almost finalised the interface design which includes all user controls and a 7 inch screen monitoring vital clinical parameters. Our next steps are to assemble and fit the prototype components to the enclosure and start the clinical testing and debugging phase.”
Regarding specific challenges to the project, Mr De Jongh notes: ”Our most glaring challenge is probably our reduced capacity for physical meetings and lab work on the project during this time. Doing this project remotely and with minimal physical contact sessions has been a challenge so far. Ordering parts from suppliers that are very low on stock and with limited operating hours has been hard.”
There are other foreseen engineering challenges, namely:
- Fitting sensors to existing ventilator and low cost medical piping (to keep product costs low).
- Designing the final effector and cradle to be up-scaled for production by potentially using injection moulding.
- Designing in a short space of time a production-ready enclosure casing, buttons, and other components.
- Identifying and establishing a medically licenced (SAHPRA/ISO13485, etc.) production partner/s.
- Performing required testing and passing relevant standards as required by SA law.
- Obtaining fundingto build and test further prototypes, manufacture samples and pre-production runs as well as first production run.
Mr De Jongh continues: “We are currently working in collaboration with Prof Andre Coetzee (retired professor in Anaesthesiology and Pulmonology at Stellenbosch University Faculty of Medicine and Health Sciences) as well as Pieter Uys, an alumnus of the Faculty of Engineering and currently at Remgro Ltd. The rest of the work is conducted by staff and students in the Department of Mechanical and Mechatronic Engineering (as part of the IBE). We aren’t currently formally collaborating with any industry partners, except for the assistance from CHG Engineering with the enclosure design.
“We hope to conduct our first proof of concept testing using an artificial test lung within the next two weeks together with Prof Andre Coetzee. After this we will refine our prototype and start with the final design, all whilst conducting continuous tests to make sure that we are satisfying basic clinical requirements for a ventilator,” he concludes.
See Video of the first test-phase.
Description: The first test-phase prototype of a low-cost Non-invasive Ventilation System (NVS) in development by Stellenbosch University’s Faculty of Engineering as part of the Institute of Biomedical Engineering (IBE). In this short video, the NVS is connected to a test lung that can be used to evaluate the ability of the device to deliver and control, within preset bounds, the main clinical parameters required from such a device.
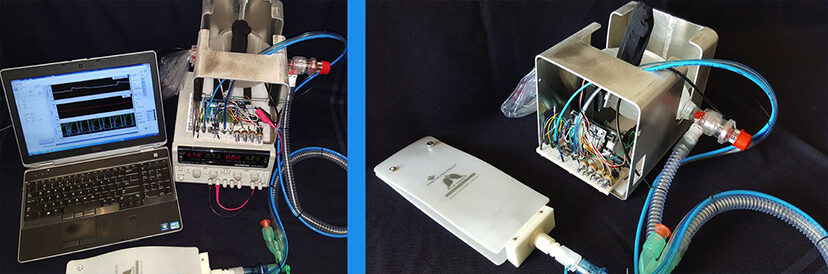
Photo Left: Ventilator prototype setup for experimental testing and verification.
Photo Right: Ventilator prototype connected to an artificial test lung for validation of clinical parameters.



